That’s a really interesting study. Large person-to-person variance in blood glucose response to specific foods (e.g. one person’s blood sugar might spike with white bread, but no whole wheat and vis-versa). The authors also wrote a book on their work, which I found quite interesting and a very quick read.
@gedankenstuecke, thanks for pointing me to this thread. It’s really exciting to see people interested in using CGMs to study what impacts their blood sugar. I’d love to participate as well.
I’ve got Type 2 diabetes and have recently gotten involved in more rigorous self-tracking and self-experimentation. I’m particularly interested in N>1 self-experiments to try to better understand person-to-person variations and be able to study interventions that are difficult to disentangle background in N=1 studies. Over on Reddit, I’m working with a group of diabetics studying the effect of hot showers on BG (protocol & preliminary). If any of you are interested in participating, we could really use data from some non-diabetics.
In terms of this thread, I agree that starting with food effects on glucose would be the easiest and quickest way to get useful data. I’m open to anything people want to try out, but one of the questions I’m most interested is the interaction effects between different macronutrients. There’s lots of small studies claiming that increasing fiber or fat in a meal slows glucose metabolism and reduces BG spikes, but they’re all observational, small sample sizes, and/or use fairly complex meals with lots of ingredients making it hard to be sure of what’s going on. I’d be interested in doing a study where we isolate the effects of consuming individual ingredients (e.g. pure sugar, protein isolate, oil), then mix and match to get a clean measure of the interaction effect.
Just a thought and as I said, I’m open to trying anything the group is interested in studying.
I’m starting some experiments to measure the effect of different ingredients and ingredient combinations on my blood glucose.
Would love some feedback on the experimental design and/or other experimenters to join in!
Yesterday, @MichaelR and I sat down to come up with an experimental plan for ourselves. So far we’ve stuck to an easy experimental plan that should be able to be performed within a 14 day (single FreestyleLibre sensor) period.
Interventions we plan:
- After waking up: Eating 3, 4 or 5 cubes of sugar with at least 1h time after taking in the sugar cube before having actual breakfast. For each of the 3 quantities we will get at least 4 glucose response curves over the 14 day period.
- For dinner: Having a meal that is either: carbs, protein or carb+proteins. Again, 3 different conditions would mean having at least 4 data points for each category. We’d also shuffle these against the breakfast conditions, in order to not have a fixed correlation with those.
It’s pretty simplistic, but maybe a good starting point? @skaye Given that you’re clearly more expert on this we wondered though whether it might be worthwhile for us all to join forces and follow one shared protocol in order to maximize our learning? Would you be interested in that? 
@gedankenstuecke, I’d love to join in! A few thoughts on the protocol:
Sugar cubes weigh ~2.3g, so this would be 7, 9, and 12 g. For a non-diabetic adult, a 75 g glucose tolerance test results in an increase of <50 mg/dL (2.7 mmol/L) after 2 h. If dose-response was linear, that would mean the 12 g dose would produce a 2 h response of <8 mg/dL (0.44 mmol/L), and that’s if you were borderline pre-diabetic. Actual response should worse as sugar cubes are solid sucrose (lower glycemic index than dissolved glucose), dose-response is less than linear for non-diabetics, and you’re unlikely to be so close to the pre-diabetes threshold for glucose tolerance. You might have a shot of seeing something in the 30-60 minute range, but I wouldn’t be confident in that.
If you want to see any effect, I’d suggest: 1) A wider range of carbs, maybe 10, 20, 40g (40 is the amount in one can of soda), and increase from there if you don’t see a measurable signal? 2) Use glucose dissolved in water (available from any pharmacy or online, faster impact on blood sugar and removes rate of eating as variable). 3) Do the experiment at some time other than the morning (your insulin sensitivity is lower in the morning and your liver dumps glucose into your bloodstream. This effect is variable depending on time you woke up, level of activity, and a bunch of other factors which will introduce noise into the experiment)
For this part of the experiment, I’d be able to do the same protocol, but would need to use a lower amount of glucose. Even 10g would raise my blood sugar by 50-60 mg/dL, which would not feel good. I would use 2, 4, and 6g to try to limit myself to a 35 mg/dL rise.
To get an interpretable result from this, you’re going to need to hold something constant (e.g. calories, mass, etc.). What are you trying learn and/or what hypothesis are you trying to test? For me, I’d be curious to test if eating carbs + protein together reduces the glucose response from the carbs (review for dairy proteins) To do this, we’d want to fix the total amount of carbs and protein and pick an amount of carbs that would produce a measurable BG spike.
Again, I’d need to pick a different amount of carbs & protein to stay in the same effect size, but totally doable.
Thanks so much for all of your feedback!
Actually, based on your experience we thought that maybe it should be us joining your efforts! And following this feedback that seems even more relevant! 
The range was set rather low, to allow non-diabetic and (pre-)diabetic people to participate in the protocol without putting anyone at risk. But maybe the approach we took is not useful as the response for non-diabetics would be too small? As a non-diabetic I tried some sugar cube eating (or rather dissolving varying amounts of them in my coffee) and could see varying spikes in my data. But maybe it’s more useful to escalate more quickly and ask people to stop increases once they hit a threshold?
We had assumed that in the morning might be best to have a stable BG baseline, not being aware of the other factors that make this a bad setting!
That works too  . I’ve started the protocol I posted. I’m currently on the second of my fasting trials to establish the right time of day for me to run the experiments, but it’s looking like early afternoon is optimal for me (probably ~1p, will post data and make a decision by Sunday). You could do the same time, but it would require skipping lunch. Since your blood glucose is a lot less sensitive than mine, I think any time that’s at least 4 hours after a meal and is consistently stable based on your CGM data would be fine.
. I’ve started the protocol I posted. I’m currently on the second of my fasting trials to establish the right time of day for me to run the experiments, but it’s looking like early afternoon is optimal for me (probably ~1p, will post data and make a decision by Sunday). You could do the same time, but it would require skipping lunch. Since your blood glucose is a lot less sensitive than mine, I think any time that’s at least 4 hours after a meal and is consistently stable based on your CGM data would be fine.
I think this is the right way to go (escalating dose until you hit a threshold). Responses between people vary wildly (even within diabetic/non-diabetic) and the we have a more narrow window in-between large enough to see above sensor noise, but small enough not to cause symptoms. I would suggest the following protocol:
-
Start with a dose of glucose that you suspect will cause a measurable increase in BG
-
If you observe an increase in BG, test linearly increasing amounts until you see a BG response of >36 mg/dL (2 mmol/L) and have at least 3 data sets with measurable BG rise.
-
If you don’t see a BG response, increase the amount of glucose geometrically (2x each time) until you do.
From this protocol, we’ll get a dose-response function for each person (i.e. BG = f(glucose consumed, t)), which will allow us to look at person-to-person variation, serve as a baseline for comparison with anything else we eat, and a baseline for comparison for any future interventions we want to study.
Yeah, I don’t know how large an effect it is for non-diabetics, but for diabetics, it’s a real pain. Means I need an extra dose of insulin and that I have to calibrate my insulin doses separately based on time of day…
Two more questions:
-
Do you want to just join in for measuring the glucose effect or other foods/ingredients as well?
-
Are you ok if I try to recruit some others to join in? I think there are some people I’ve been working with on the shower effect study who might be interested and I’m curious to see what person-to-person variation looks like on the more complex ingredients. Segal and Elinav have published several papers showing that BG response to foods varies relative, not just absolute magnitudes. I’m curious to see if it replicates under more controlled conditions and to get a better measure of the effect size.
thanks for the protocol hints above. I think for now we’ll just try to follow the glucose effect I’d say. But I’ll check in with @MichaelR!
I think that would be great, moving from the self-experimentation to co-created group-experiments is fantastic to see!
Great! I’ll send out some recruiting posts over the weekend and reach out specific individuals I think might be interested.
BTW, I finished collecting my fasting baseline (will add report over the weekend). Looks like I’m stable around 12p, so I’ll start the glucose measurements tomorrow with a 4g dose (should raise me ~22 mg/dL). I’ll let you know how it goes.
That’s awesome! I just ordered a couple of sensors so that we can get started with our own contributions!
To give a progress update as well:
I finally installed my sensor yesterday morning, to give it a 24h window to become less weird, as the Libre sensors seem to virtually not produce any signal in this period.
This morning (9:50am, like 10 minutes ago) I went ahead and did a first testing measurement using sugar cubes: I dissolved 6 sugar cubes of 6 grams each in a glass of warm water, coming in for 36 g of sucrose (Despite loving sugary things in all forms, I have to say this was rather strong even for my taste  ).
).
I decided to stick to sucrose for now instead of going for glucose largely out of laziness: Sugar cubes are easy to get in any supermarket and are easy to dose in increments of 6g 
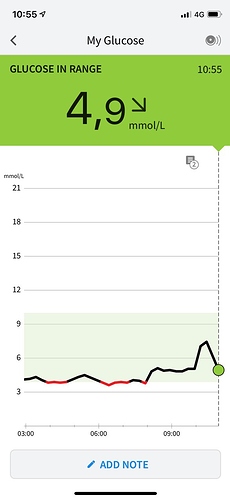
I forgot to post it earlier, but this was the situation ~1 hour after the 36 g of sucrose, more or less being back to normal.
Well, you definitely don’t have diabetes  .
.
I’d be really curious to see how this varies with the amount of sugar consumed. In my experiments, I’m tracking (all vs. grams of food consumed):
- peak increase in blood glucose
- incremental area under the curve
- change in blood glucose after 4.5 h (almost certainly irrelevant for someone without diabetes).
I found the first two to be virtually linear with amount consumed for a net effect of 0.37 mmol/L/g(glucose) (or 6.7 mg/dL). That would mean that if I ate the same 36 g that you did, my blood glucose would spike by 13.4 mmol/L (a bit less b/c sucrose is slower to digest than glucose).
What are you going to try next?
So far I did two more replicates of the same amount of sugar, both are around 10am in the morning. In both cases the peak was at 7.x mmol/L. On Sunday and today I missed the time for doing the next data collection, but I hope to take a larger amount of sucrose for 3 days to see how the response changes!
I’d be interested to see how interactions e.g. with protein work out though, if you have tips there? 
Great!
I’ve recently been reading up on effect of protein and amino acids on blood sugar.
There’s a fair amount of literature on the topic. Here’s two articles I found particularly useful: review, article discussing timing effect. Happy to send more if you’re interested.
The upshot is that lots of studies have observed reduced blood sugar response to glucose when protein or amino acids were consumed before or at the same time. The mechanism is not as clear, with some studies showing increased insulin response, some showing increased insulin sensitivity, and some showing both.
Procedures vary, but a common one is to take 150 mg/kg or 1 mmol/kg of protein either with or 30-60 min. before consuming a set amount of glucose. Once I get to combination studies, I was planning to do that and test a few different protein quantities and time lags.
Given that France just announced a total lockdown for at least 15 days, getting groceries to test might be harder than expected. In that case I’ll just keep fumbling around with different sucrose levels. 
Yikes. The Bay Area just got a lockdown order as well (through 4/7), but I think I can still get deliveries. Either way, my plan is to get creative with what I have on hand.
BTW, I’m running a test with 100 g of oat fiber as we “speak”. 75 min. in and virtually zero blood sugar response. Really glad to see that, as I use this in a lot of my recipes.
Planning to try cooked out fiber on Wednesday to see if that makes it more digestible. Will share a write up over the weekend.
If you have honey, flour, or cornstarch, might be interesting to try other pure carbohydrates with lower glycemic index than sugar.
I’ve clearly been away from the Bay Area (and the US in general) for too long, as I parsed that date as 4th of July and was wow, you folks are getting serious!! 
To the topic at hand: I should have flour, cornstarch and honey around and can give that a try!